以下文章转载自:
American Journal of Neuroradiology
Presurgical Mapping with fMRI and DTI: Soon the Standard of Care?
The technique of fMRI has been around for over 30 years, and DTI for about 15 years. The first application of fMRI was by Ogawa et al, in 1990. In a rat model, this team was able to manipulate the blood oxygen level–dependent (BOLD) signal by inducing changes in deoxyhemoglobin concentrations with insulin-induced hypoglycemia and anesthetic gases. About a year later, Kwong and Belliveau published the first images of cerebral areas that responded to visual stimulation and vision-related tasks.
DTI was first described by Basser et al, who were experimenting on a voxel-by-voxel characterization of 3D diffusion profiles, which took into account anisotropic effects (instead of eliminating them, as in standard DWI). Tractography (or fiber tracking) was developed by applying statistical models to DTI data to obtain anatomic fiber bundle information.
Although both fMRI and DTI are now currently available in most scanners, well beyond the framework of academic institutions and research protocols, these techniques are not quite considered “standard of care.” Indeed, the processes that govern the translation of new technology into clinical practice are complex. Even more complex are the processes that lead to establishing clinical practice as standard of care, particularly at a time when established patterns of care delivery are being increasingly challenged and economic difficulties affect all aspects of society, certainly including health care.
However, some challenges, especially with fMRI, go back to basic cerebrovascular physiology. The cerebrovascular response to neuronal activation, also referred to as “functional hyperemia,” was first recognized in 1890 by Roy and Sherrington, who initially proposed a metabolic hypothesis to the phenomenon, ie, mediation via release from neurons of vasoactive agents in the extracellular space. The major role of astrocytes as key intermediaries in the neurovascular response — being interposed between blood vessels and neuronal synapses via their foot processes as modeled in the “tripartite synapse model” of the neurovascular unit — has since been recognized. Although complex, astrocyte response to changes in synaptic activity is primarily mediated by glutamate receptors through changes in intracellular Ca2+ concentration.
In fMRI, contrast is based on the BOLD effect, which reflects local shifts of deoxygenated-to-oxygenated hemoglobin ratios due to local increases in blood flow in excess of oxygen utilization following brain activity. As a result, the foundation of the fMRI BOLD signal is based on local changes in cerebral blood flow that are not linearly related to the metabolic changes inducing the flow change.
Therefore, BOLD fMRI rests on 3 major approximations: 1) the technique does not directly reflect neural activity, ie, generation and propagation of action potentials, synaptic transmission, or neurotransmitter release/uptake; 2) the changes in BOLD signal originate from that portion of the vasculature experiencing the greatest change in oxygen concentration, which occurs in the venules in the immediate vicinity of the active neurons; and 3) more importantly, fMRI signal relies on intact “neurovascular coupling,” the phenomenon that links neural activity to metabolic demand and blood flow changes.
The main reason fMRI is clinically useful most of the time is that under most circumstances neurovascular coupling remains fully intact, unaltered by confounding disorders that can interfere with this relationship. However, it has long been known that neuronal activation results in local blood flow increases that exceed local oxygen consumption, so that the oxygen utilized may constitute a small fraction of the amount delivered. Under normal conditions, the oxygen concentration in draining venules increases during neuronal activation. The original researchers who discovered this phenomenon named it “neurovascular uncoupling” or “neurovascular decoupling.” From a medical perspective, “uncoupling” or “decoupling” implies a pathologic condition, suggesting something abnormal about tissue that demonstrates this phenomenon. More recently, researchers have preferred the term “functional hyperemia” to describe the phenomenon. In fact, when there is interference with the mechanism producing functional hyperemia, the term "neurovascular uncoupling" has been re-applied, albeit with a completely opposite meaning from that originally used. Impairment in the flow response leads to neurovascular uncoupling and a reduced BOLD signal in response to neural activity, which can lead to false-negative errors in fMRI maps.
John Ulmer, reporting on a series of 50 patients, found that although accurate cortical activation could be demonstrated most of the time, various cerebral lesions could cause false negatives in fMRI results when compared with other methods of functional localization, suggesting contralateral or homotopic reorganization of function. He further suggested that pathologic mechanisms such as direct tumor infiltration, neovascularity, cerebrovascular inflammation, and hemodynamic effects from high-flow vascular lesions (ie, arteriovenous malformations and fistulas) could trigger “neurovascular uncoupling” in those patients. Neurovascular uncoupling, and other pitfalls of fMRI, are briefly discussed.
David Mikulis discusses “neurovascular uncoupling syndrome,” where lack of functional hyperemia during neuronal activation can have long-term consequences on the integrity of the tissue in the absence of acute ischemia.
Jay Pillai discusses the successful clinical application of a technique to improve the consistency of BOLD fMRI by using a breath-holding technique.
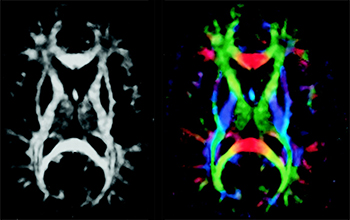
Aaron Field discusses the technique, clinical use, and some limitations of DTI and tractography, and describes patterns of alteration of white matter fiber tracts by neoplasms and other lesions.
Lastly, Wade Mueller shows that a neurosurgeon may obtain significant improvements in clinical outcomes and a drastic reduction in complication rates when working with a team that provides presurgical mapping of cerebral lesions by using fMRI and DTI (wisely, fully acknowledging their limitations) and when various team members clearly communicate using a common language.
Functional MRI and DTI are extremely useful techniques that have become increasingly available to neuroradiologists in recent years. As with any technique, these work best as parts of a whole. A good understanding of physiologic mechanisms is necessary to make us good “functional” specialists, and a good understanding of the limitations of any technique is necessary to make us better physicians.
Image modified from: Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns.
春潮启序,喜乐相伴;凝心聚力,共赴新程。2026年2月6日,美德医疗年会暨惠州龙门团建活动在惠州自然谷温泉民宿隆重举行,全体美德家人欢聚一堂,共庆2025年丰收硕果,共绘2026年发展蓝图。现场暖意融融、欢声雷动,每一份喜悦都源于过往的深耕不辍,每一份憧憬都承载着未来的无限可能。 回望2025年,是美德医疗砥砺奋进、再创辉煌的一年。在全体同仁的同心同德、奋力拼搏下,公司销售额突破历史新高,交出了一份亮眼的发展答卷,这份成绩的背后,是每一位美德人日复一日的坚守与付出,是不分岗位、不分昼夜的担当与耕耘。当业绩捷报传来,现场掌声雷动、欢欣鼓舞,所有的疲惫都化为满满的成就感与自豪感,大家深刻体会到,每一份努力都不会被辜负,每一次坚持都终将收获回响。 年会现场,议程紧凑而隆重,既是对过往的全面复盘,更是对未来的明确指引。大会伊始,总经理汤洁女士发表开幕致辞,她首先向全体员工致以最诚挚的新春问候和最衷心的感谢,全面回顾了2025年公司在医疗设备研发、市场拓展等领域取得的突破性成果,深情肯定了各部门的辛勤付出与全体同仁的敬业奉献。汤总强调,年会不仅是一场庆功盛宴,更是一次凝心聚力、谋篇布局的重要契机,它承载着复盘过往、表彰先进、明确方向、凝聚共识的重要意义,让每一位美德人都能在回望中坚定信心,在总结中汲取力量,在展望中明确目标,为公司持续深耕医疗领域、实现高质量发展筑牢思想根基与团队根基。 致辞结束后,各部门依次上台,全面回顾了2025年的工作亮点、成长收获与不足改进,同时立足公司发展战略,清晰规划了2026年的工作重点与实施路径,展现出各部门凝心聚力、奋勇争先的昂扬姿态。 随后,副总及科研合作专家吴博士发表讲话,结合行业发展趋势与公司科研布局,为大家分享了前沿理念与发展思路,鼓励全体同仁持续创新、精益求精,在医疗科技赛道上勇攀新高,为推动行业进步贡献美德力量。 最后,总经理汤洁女士进行点评总结,既肯定了各部门的工作成效,也对新一年的工作提出了更高要求与殷切期望,激励全体同仁不忘初心、牢记使命,以更饱满的热情、更务实的作风,共同书写美德医疗新的辉煌。 表彰环节是年会的重要亮点,更是对每一份付出的最高致敬。为树立榜样、激励先进,公司对2025年度表现突出的优秀团队与先进个人进行了隆重表彰,总经理汤洁女士为优秀员工一一颁发奖牌和奖金。 最令人期待的现金抽奖环节,将现场气氛推向第一个高潮。南昆山的自然谷民宿会场,惊喜不断、欢呼连连,每一份奖项都承载着公司对全体员工的关爱与回馈,让大家在收获快乐的同时,更感受到了美德大家庭的温暖。 年会落幕,欢乐不散。当晚,自助烧烤、篝火晚会如期举行,正式开启了轻松惬意的团建时光。滋滋作响的烧烤香气扑鼻,大家围坐在一起,一边享用美食,一边开怀畅饮,一边畅谈工作与生活,欢乐的氛围让人感到从未有过的放松。 篝火熊熊燃烧,大家手拉手围着火炉载歌载舞,放声欢笑、尽情释放;露天K歌环节中,同事们一展歌喉,用歌声诉说心声、传递快乐;欢乐麻将、桌球、篮球等休闲项目更是热闹非凡,大家踊跃参与,在欢声笑语中重温学生时代的纯粹与快乐,在轻松惬意的氛围中彻底放松身心,享受着难得的休闲时光。 次日,丰富多彩的团建游戏正式拉开帷幕,趣味十足、兼具协作性与挑战性的游戏项目,让大家在欢乐中收获成长,在协作中领悟真谛。在游戏过程中,大家相互鼓励、相互支持,发挥集体智慧和力量,在欢声笑语中深刻领悟到了集体精神与智慧力量的重要性——正如团建活动的核心意义,它不仅是一次身心的放松,更是一次团队精神的锤炼与融合,让大家在轻松的氛围中深化彼此了解、增进团队信任、培养协作意识,明白个人的力量有限,而集体的力量无穷,只有心往一处想、劲往一处使,同频共振、协同发力,才能克服一切困难,实现个人与公司的共同成长。 团建游戏结束后,游泳PK环节顺势登场,成为整场团建的又一大亮点,总经理汤洁女士主动邀约两位同事同台竞技,现场氛围瞬间升温。PK过程中,总经理泳姿标准规范、动作流畅舒展,速度丝毫不见逊色于年轻人,展现出昂扬向上、不服输的精神风貌;年轻的00后同事虽泳姿不及众人专业,但凭借充沛的活力与强劲的爆发力,一路领先;研发部的周工也不甘落后,奋力追赶、全力以赴,每一个划水动作都尽显拼搏姿态,三人的精彩比拼赢得岸边同事阵阵呐喊喝彩,既增添了团建的趣味性,也展现了美德人奋勇争先、永不言弃的精神。 欢乐的时光总是短暂,为期两天的年会暨团建活动在欢声笑语中圆满落幕。此次活动,既让全体同仁在庆功盛宴中感受到了公司的关怀与温暖,在休闲娱乐中彻底释放了工作压力,更在团建协作中凝聚了团队力量、锤炼了团队精神,深刻诠释了“同频共振,协创未来”的活动主题。 旧岁已展千重锦,新年再进百尺竿。2025年,美德医疗圆满收官,用汗水浇灌收获,用奋斗书写辉煌;2026年,新的征程已然开启,新的使命催人奋进。 此次年会暨团建活动,不仅是一次总结过往、庆祝丰收的盛会,更是一次凝心聚力、扬帆起航的动员令。相信全体美德人将带着此次活动中的欢乐与收获、信心与力量,不忘初心、奋勇争先,秉持“客需为本,品质为先;诚信友善,共创辉煌”的核心价值观,以更饱满的热情、更务实的作风、更创新的精神,同心同德、同频共振,在医疗科技领域持续深耕、奋勇前行,共同谱写美德医疗更加灿烂美好的明天!
当磁共振成像(MRI)技术不停迭代创新,作为临床诊断和科学研究的工具利刃愈加锋利之时,高达 110-130 分贝的扫描噪声——相当于飞机起飞时的轰鸣声——仍旧是 MRI 扫描中难以完全克服的问题。剧烈的噪声不仅会给被试带来不适,引发焦虑情绪,更会掩盖扫描室内外的有效沟通。扫描进程中,通过耳塞、软垫等物理降噪以及佩戴耳机,被试仍可获取外部声音,但被试的实时语音则完全湮没在强噪声中,无法清晰传递给操作人员;这一方面会导致无法采集被试的语音反馈、行为指令交互等数据,另一方面也会直接妨碍某些紧急情况下的应急处理。试想,当磁共振扫描中的语音沟通不再有障碍,将会有多少新的可能!破解磁共振扫描沟通难题:美德医疗核磁语音降噪系统针对这一行业痛点,美德医疗重磅推出核磁语音降噪系统—— 一款专为MRI强噪音环境设计的磁兼容语音系统,通过技术创新,将智能硬件和自适应算法相结合,从底层解决扫描过程中的被试语音数据撷取问题。该系统的功能亮点如下:强效降噪,还原纯净语音:系统可抑制扫描噪声高达40dB,有效提取并还原被试原始语音,确保声音信号的高保真、高信噪比,为语音数据质量提供坚实保障。广谱降噪,多序列适用:不同于只针对脑功能成像序列(EPI)进行降噪的竞品,本系统具备广谱降噪能力,可根据用户需求拓展至所有磁共振机型与扫描序列,满足科学实验与临床应急的多元需求。灵活部署,即插即用:系统采用模块化设计,为独立产品形式,轻松实现扫描室内外双向清晰对讲。也可与美德脑功能视听觉刺激系统无缝对接,快速升级现有实验平台,提升研究灵活性。功能拓展,助力深度科研:支持外接USB、蓝牙、MP3等多种音源,具备双通道(降噪前后)语音同步采集功能,完整留存语音数据,支持后续精细化分析与学术输出。在认知神经科学、脑科学、心理学等研究中,清晰可靠的语音交互是实现复杂实验范式的关键。本系统有效攻克磁共振噪声干扰下的语音采集难题,为需要语音反馈的实验提供可靠技术支撑,从而显著提升数据质量与研究结果的可信度,赋能前沿科学探索。
继上半年亮相神经科学、影像技术领域学术舞台、彰显行业担当后,美德医疗在 2025 年 11 月持续以 “学术参与者”“行业协同者”“人才赋能者” 的三重身份活跃于交流前沿。短短一月内,团队跨越济南、北京、长沙、宁波四座城市,接连登陆心理学、认知科学、放射医学、磁共振影像四大领域全国性学术盛会,同步落地专业技能培训班,以深度交流促协作、以思想碰撞谋发展、以学术赋能育人才,全方位践行对多学科医疗领域进步的持续关注与积极推动。第一站:济南 | 第二十六届全国心理学学术会议(10.31-11.2)作为国内心理学领域规模最大、影响力最广的学术会议之一,第二十六届全国心理学学术会议汇聚了来自全国高校、科研院所及临床机构的数千名心理学专家学者,围绕 “心理学研究创新与社会服务价值” 展开深度研讨。美德医疗团队全程深度参与本次盛会,不仅积极融入 “科研协作生态构建”“心理学研究范式升级” 等核心分论坛,更与众多专注于脑认知、临床心理学研究的学者展开一对一交流。双方就 “跨机构研究数据协同”“心理学实验场景优化” 等行业痛点交换见解,为后续搭建心理学领域科研协作桥梁、推动研究资源高效整合奠定了良好基础。展会现场,美德医疗对心理学研究领域的关注与投入,也赢得了参会嘉宾对美德医疗品牌的高度认可。第二站:北京 | 中国认知科学学会认知与计算关系问题研讨会(11.6-7)在认知科学与计算科学深度融合的趋势下,中国认知科学学会主办的 “认知与计算关系问题研讨会” 成为领域内聚焦前沿交叉话题的核心平台。本次会议邀请了认知科学、计算机科学、神经科学等多领域专家,共同探讨 “认知机制的计算建模”“跨学科研究方法创新” 等关键议题。美德医疗创始人汤洁女士深度参与其中,重点围绕 “认知科学研究的临床转化路径”“多学科数据整合应用” 等话题分享实践洞察,探讨如何打破学科壁垒、推动认知科学研究成果更好地服务于临床需求。此次参会不仅让美德医疗更精准地把握了认知与计算交叉领域的发展方向,也为公司后续参与跨学科协作、助力认知科学临床落地积累了宝贵经验。第三站:长沙 | 中华医学会第 32 次放射学学术大会(11.13-16)作为放射医学领域的年度 “学术盛宴”,中华医学会第 32 次放射学学术大会吸引了国内外数万名放射科医师、科研人员及行业从业者,聚焦 “智能放射发展”“临床质控标准化”“放射技术临床应用升级” 三大核心方向,是推动我国放射医学领域进步的关键平台。美德医疗携近期研发新品亮相,通过展位交流、圆桌座谈等形式,倾听临床一线对放射医学领域发展的需求与期待,进一步明确了品牌 “以临床需求为导向、助力放射医学进步” 的发展方向。美德医疗对放射医学领域长期的关注与支持,也让更多行业同仁看到了品牌与领域共成长的决心。第四站: 中国体视学学会磁共振成像分会 2025 年会暨第二届诺丁汉高级磁共振成像论坛(11.21-23)会议以"磁聚·共创·振新"为主题,吸引了来自全国高校、科研院所、医疗机构及知名企业的500余位专家学者、临床医师和青年科研工作者参会,共同研讨磁共振成像领域的前沿进展与创新生态建设。通过高水平的学术交流、创新的组织形式和深度的产学研合作,有效推动了磁共振成像领域的协同创新。美德医疗深度参与交流,紧密协同体现了学术界与产业界联合发展,为构建我国磁共振成像技术创新生态体系积极贡献自己的一份力量。学术赋能不停步:美德医疗第 16 届 Task-fMRI 基础培训班在密集参与学术展会的同时,美德医疗持续深耕学术赋能,全程负责运行服务的深圳第 16 届 Task-fMRI 基础培训班同步落地。作为延续多年的品牌学术支持项目,本次培训班聚焦 Task-fMRI 技术的基础理论与实操应用,课程设置兼顾 “理论深度” 与 “实践适配性”,汇聚领域资深专家授课,为来自全国高校、科研机构的学员提供从实验设计、数据采集到结果分析的全流程指导。美德医疗团队以专业的组织服务保障培训顺利开展,助力学员快速掌握 Task-fMRI 核心技术,为行业培养兼具理论素养与实操能力的专业人才,持续为磁共振相关科研领域注入新生力量。四展连轴 + 学术赋能,初心如磐筑生态从心理学的科研协作、认知科学的跨学科融合,到放射医学的临床进步、磁共振成像的技术深耕,再到 Task-fMRI 领域的人才培育,美德医疗 11 月的学术之旅,始终以 “推动领域发展、促进行业协作、培育专业人才” 为核心。不局限于单一领域,不止步于表面参与,而是深入交流核心、倾听一线需求、分享实践见解、搭建协作桥梁、赋能行业人才,这正是医疗企业 “助力行业进步” 的责任践行。未来,美德医疗将继续以学术交流为纽带、以人才培育为支撑,持续关注多学科医疗领域动态,以更积极的姿态融入行业生态,与全球医疗同仁携手,为医疗创新发展、为更好服务患者贡献 “美德力量”!关注我们,获取更多医疗领域学术动态与品牌实践,期待与您在更多学术舞台相遇,共探医疗未来!
当深圳海风遇见成都烟火,当北京沉稳碰撞川蜀灵动,10 月 25 - 29 日,美德团队一行 47 名来自深圳、北京、成都三地的同事跨越山海,开启为期五天的川蜀团建之旅。从千年文明遗址到人间仙境,从红色初心之地到烟火市井街巷,这场兼具文化深度与团队温度的旅程,让大家在放松身心的同时,更让 “协作共赢、互帮互助” 的企业文化深深扎根。团建首站直奔三星堆遗址,青铜神树的瑰丽、纵目面具的神秘,同事们纷纷驻足惊叹。“沉睡数千年,一醒惊天下”。三星堆遗址作为中国古代文明的重要遗址之一,印证了中华文明起源的多元性。文明探源,在传承中凝聚默契,同事们第一站即为之深深触动。次日抵达都江堰,这项举世闻名的世界遗产工程,是人类和自然和谐共生的典范。站在鱼嘴分水堤上,两千多年来滋养成都平原的岷江碧水,奔流而下,大家不由得感概万千,折服于古人 “因势利导”、“道法自然” 的智慧。此次行程中,公司特意安排了汶川地震博物馆这一站,为的是让大家铭记历史,珍惜生命,关爱他人。如今站在映秀这片宁静祥和的土地上,抗震救灾的众志成城与灾后重建的涅槃重生,让不少同事湿润了眼眶。在纪念碑前,大家自发列队致敬,深刻体会到 “人心齐,泰山移” 的力量。九寨沟与黄龙的自然风光,成为心灵的治愈之旅。九寨沟的碧蓝海子、飞泻瀑布,黄龙的钙化彩池、层林尽染,让大家暂时卸下工作压力,沉浸在大自然的鬼斧神工中。同事们相互抓拍美景,分享观景心得,欢声笑语回荡山谷。登高黄龙途中,因雪天过后气温较低,路面结冰打滑容易摔跤,同事们几人一组结伴前行,相互提醒,互相照顾;行至海拔最高处,有的同事轻微高反,其他同事拿出自己的氧气瓶给同事吸氧;还有的同事下山后头疼,有热心的同事就拿出自己的药品给同事服用,这种团结互助的精神,在此次行程中得到了充分的彰显。松潘古城墙下,大家并肩漫步,聊着工作趣事与生活感悟,地域距离悄然消融,心与心愈发贴近,默契在同行中自然滋生。老板的精心安排,让川蜀烟火气成为团建的温暖注脚。正宗川味火锅宴上,红油沸腾,食材鲜香,同事们围坐畅饮,辣得酣畅,聊得尽兴。席间,老板特地为大家安排了变脸表演,演员精妙的技艺引来同事们阵阵欢呼,麻辣鲜香的火锅与非遗变脸的配搭,全员嗨翻,将氛围感和幸福感直接拉满。晚饭后,小伙伴们打卡玉林路的小酒馆,哼着赵雷的《成都》,在小酒馆门口合影留恋,温情满溢,向往已久的心情得到了无比的满足。最后一天,我们早早出发前往熊猫繁育基地,渴望亲眼见到明星“花花”,然而时间有限,我们未能一睹她的芳容,倒是拍摄到了她的其它家族成员。个个憨态可掬,或爬树,或酣睡,或仰卧着吃竹子,形态各异,可爱至极;或嬉戏打闹,或趴在树杈上睡觉,或坐成粽子状打盹背对观众,这身影,这背影,这造型,治愈了大家许久以来的焦虑和疲惫。五天的行程紧凑充实,身体虽累,内心却无比丰盈。所有同事始终服从安排、恪守时间,用行动诠释 “守时守信、互帮互助” 的团队准则。这场团建让跨地域同事从 “线上协作” 变为 “线下并肩”,让个体力量汇聚成团队合力。未来,美德医疗团队将带着这份友谊与信念,以更饱满的热情、更团结的姿态投入工作,秉持 “为善致乐,相互合作,共建和谐阳光团队” 的企业文化,携手书写公司发展新篇章!致敬每一位同心同行的 "美德人”
2025 年 10 月 22 日下午,深圳市美德医疗电子技术有限公司迎来市人大常委会教科文卫工委一行人莅临调研访谈,本次调研访谈聚焦于脑机接口产业立法相关事宜,旨在为该新兴产业的规范发展提供法治保障。调研访谈伊始,美德医疗总经理汤洁女士向市人大常委会教科文卫工委一行详细介绍了公司概况。作为国内脑功能成像领域的领航企业,美德医疗自 2004 年成立以来,始终专注于脑科学设备及医学影像配套产品的研发生产、技术培训与科研服务,为推动脑科学产业发展做出了积极贡献。汤洁女士还带领调研组参观了公司,全方位展示了美德医疗在技术研发、生产制造以及科研服务等方面的先进设施与专业团队,让调研组对公司有了更为直观、深入的了解。参观结束后,调研访谈正式开始。会议由市人大常委会教科文卫工委主任主持。市人大代表、市人大常委会教科文卫工委委员以及企业代表依次发言,围绕脑机接口产业立法展开深入交流与探讨。在发言中,美德医疗代表着重强调了脑机接口技术在医疗、康复等领域的巨大应用潜力以及当前产业发展的机遇与挑战,呼吁立法机关在保障技术创新的同时,关注伦理、安全等关键问题,为产业健康可持续发展奠定坚实基础。市科技创新局相关负责同志也对调研访谈中提出的问题作出回应,表示将积极配合立法工作,推动脑机接口产业相关政策的完善与落实。此次市人大常委会教科文卫工委到深圳市美德医疗电子技术有限公司进行调研访谈,不仅是对脑机接口产业立法工作的有力推进,更是对美德医疗在脑科学领域所取得成绩的充分认可。作为行业领军企业,美德医疗将继续秉持创新驱动、技术引领的理念,积极参与行业标准制定与立法研讨,以高质量的产品与服务助力脑机接口产业蓬勃发展,为深圳打造脑科学创新高地贡献更多力量。